Surgery poses a serious risk of bleeding in people with von Willebrand disease and necessitates careful monitoring and management.1
Normalising the activity of both von Willebrand factor and factor VIII is important to maintain haemostasis during surgery to minimise the risk of prolonged or excessive bleeding.1, 2 The efficacy of wilate® for surgical prophylaxis has been demonstrated in clinical trials and is supported by real-world experience for major and minor surgeries, in patients with all types of VWD. There have been no reports of thromboembolism or factor VIII accumulation in any clinical study of wilate®.3, 4
Normalisation of both VWF and FVIII to ensure normal haemostasis1
Avoidance of FVIII accumulation with repeated dosing of VWF/FVIII which may increase risk of thrombosis5
Close monitoring of VWF and FVIII levels to avoid under- or over-dosing6
* the indicated use is for prevention and treatment of haemorrhage or surgical bleeding in von Willebrand disease (VWD), when desmopressin (DDAVP) treatment alone is ineffective or contra-indicated. FVIII:C: FVIII clotting activity; VWF:RCo: VWF Ristocetin Cofactor.
Parallel decay of VWF:RCo and FVIII:C
May simplify dosing and monitoring using either VWF or FVIII
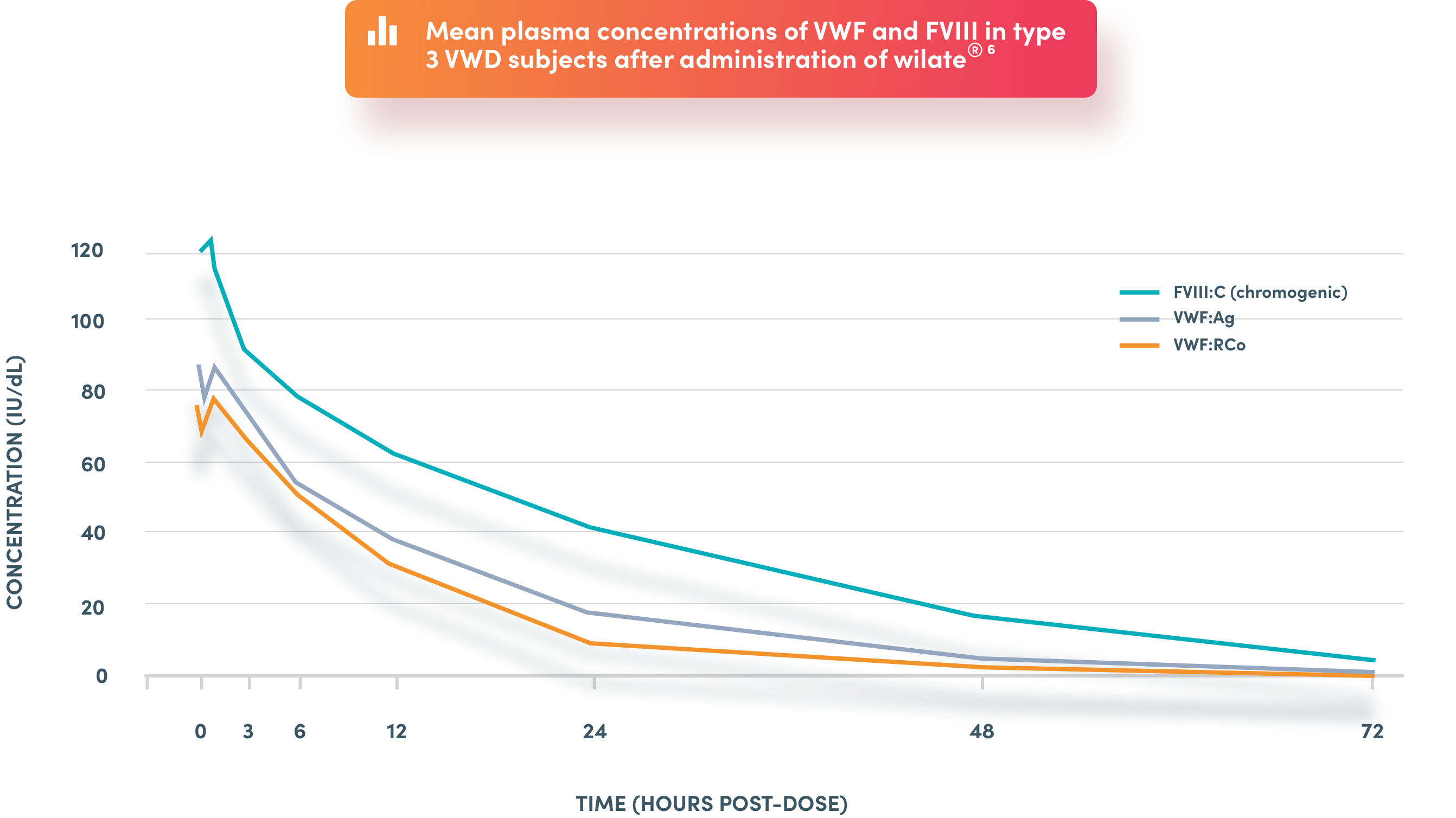
* the indicated use is for the prevention and treatment of haemorrhage or surgical bleeding in von Willebrand disease, when desmopressin treatment alone is ineffective or contra-indicated. FVIII:C: FVIII clotting activity; FVIII: factor VIII, VWF: von Willebrand factor; VWF:Ag: von Willebrand factor Antigen; VWF:RCo: VWF Ristocetin Cofactor
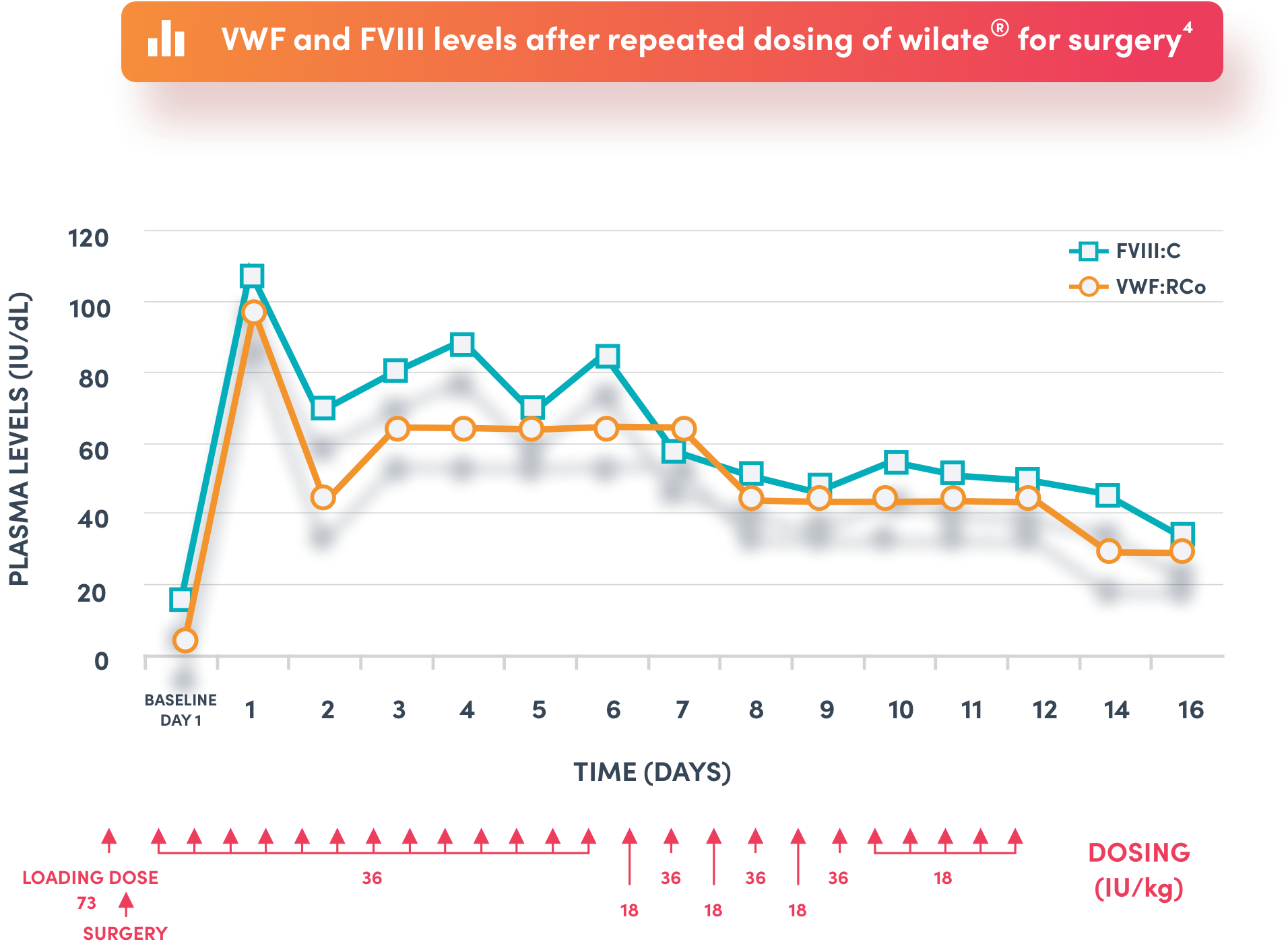
Representative recovery profile in an 18 year old female patient with type 3 von Willebrand disease undergoing leg elongation surgery4
No FVIII accumulation seen despite repeat dosing4
* the indicated use is for the prevention and treatment of haemorrhage or surgical bleeding in von Willebrand disease, when desmopressin treatment alone is ineffective or contra-indicated. FVIII:C: FVIII coagulation activity; FVIII: factor VIII and VWF: von Willebrand factor; VWF:RCo: VWF ristocetin cofactor.
* the indicated use is for the prevention and treatment of haemorrhage or surgical bleeding in von Willebrand disease, when desmopressin treatment alone is ineffective or contra-indicated. FVIII: factor VIII; VWD: von Willebrand disease
reported in any clinical study or published real-world clinical experience3, 4, 7–9
WONDERS: Prospective, open-label, uncontrolled Phase III study of wilate® in surgery
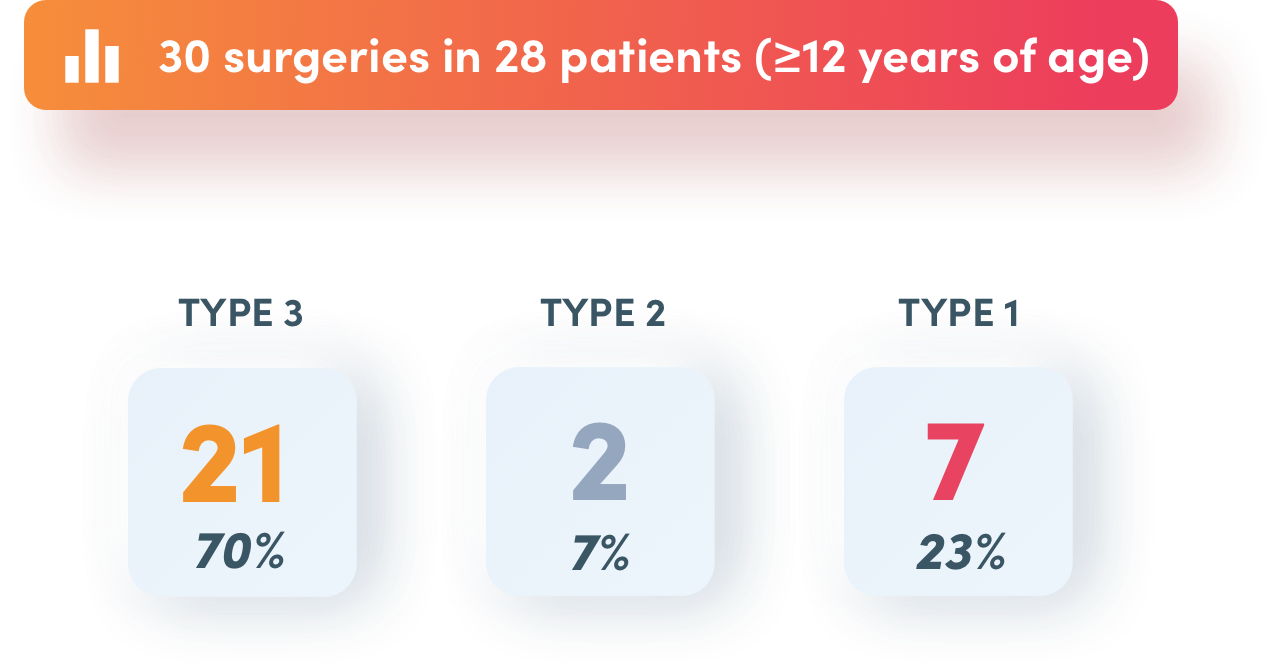
FVIII: factor VIII
Overall efficacy rated as successful* in 97% of surgeries
* IDMC-adjudicated treatment success rating based on haemostatic efficacy assessment (intraoperatively by surgeon and postoperatively by investigator) using objective 4-point scale (excellent, good, moderate, none). FVIII: factor VIII
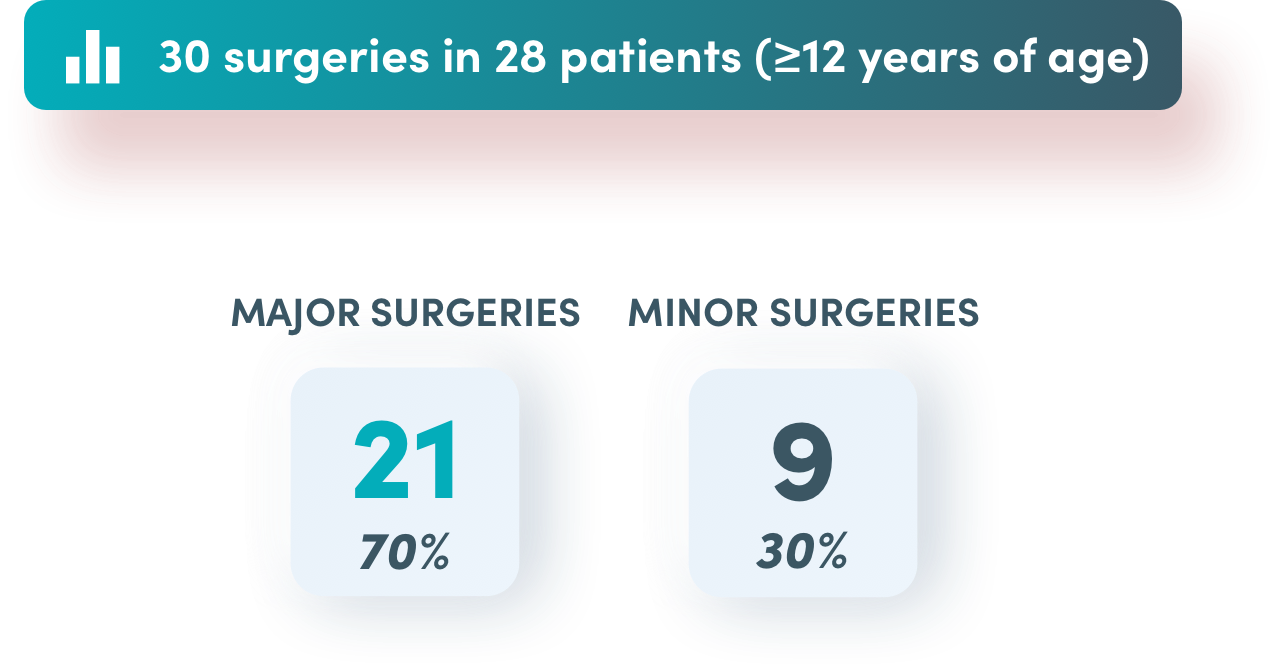
Success in major surgeries (20/21)
Success in minor surgeries (9/9)
Success in 21 surgeries in 20 patients with type 3 von Willebrand disease
Median dose per infusion of wilate®
Loading dose
Maintenance dose
FVIII: factor VIII

*0 = pre-surgery loading dose. Error bars represent standard error of the mean. FVIII: factor VIII; FVIII:C: FVIII coagulation activity; VWF:Ag: VWF antigen; VWF:RCo: VWF ristocetin cofactor.
WIL-20: Prospective, observational Phase IV study on the use of wilate® in
von Willebrand disease

*Diagnosis data not available.
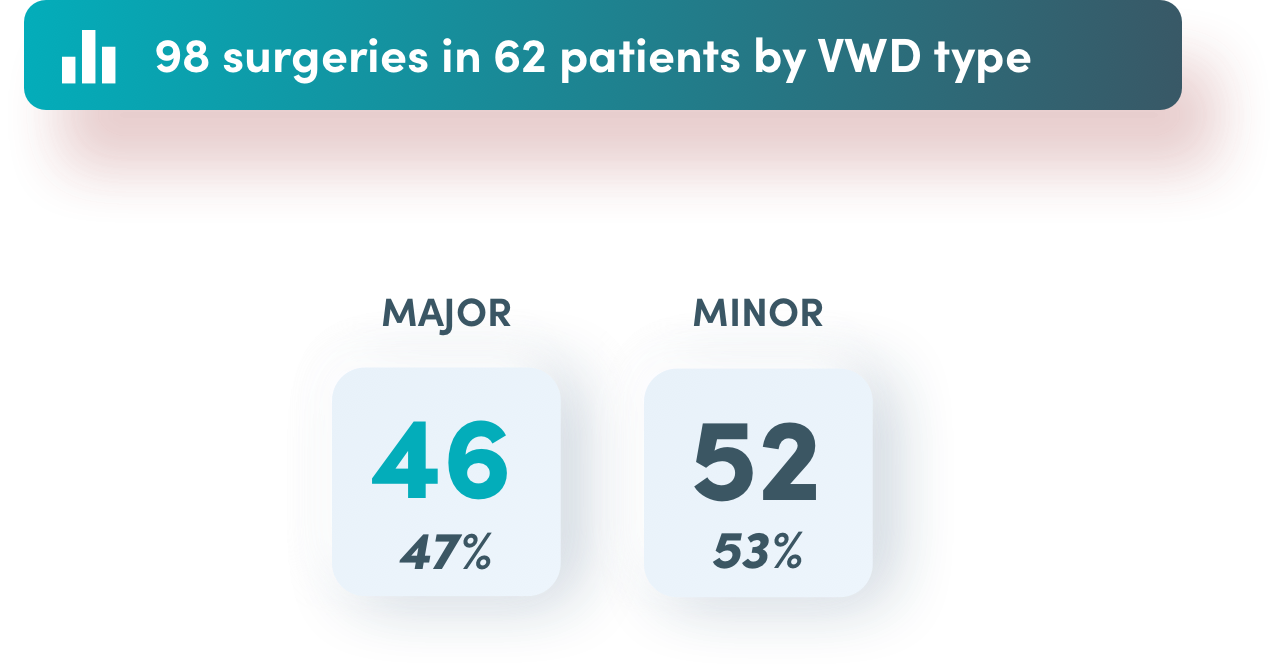
Excellent or good effectiveness in surgical prophylaxis for*
* IDMC-adjudicated treatment success rating based on haemostatic efficacy assessment (intraoperatively by surgeon and postoperatively by investigator) using objective 4-point scale (excellent, good, moderate, none).
of 46 major surgeries
of 52 minor surgeries
of all surgeries
Median dose of wilate® per procedure
*Haemostatic effectiveness assessment after surgery using objective 4-point scale (excellent, good, moderate, none). Effectiveness rating not available for one minor surgery.
for major surgeries range: (22–500 IU/kg)
for minor surgeries
range: (6–680 IU/kg)
Reported in the WIL-20 study
Information about on-demand treatment with wilate®
Prevention of bleeding with wilate®
Use of wilate® in people with von Willebrand disease

The information on this website is not country specific, and may contain information that is outside the approved indications in the country in which you are located. Please contact your local Octapharma representative for the latest information specific to your country.
Please contact your local Octapharma representative for local prescribing information via our contact form.
IMPORTANT
The information on this website is based on the European Summary of Product Characteristics (EU SmPC). US HCPs and other country-specific websites: Below is a list of countries that host a local wilate® website based on local approved information and in local language. Click on the country link to be redirected to the local wilate® website.
The website is provided by Octapharma AG, Seidenstrasse 2, 8853 Lachen, Switzerland
www.octapharma.com
© 2024 Octapharma AG