In many people with von Willebrand disease, the activity of both von Willebrand factor (VWF) and factor VIII (FVIII) are reduced.1
For effective prevention and treatment of bleeds, the activity of both clotting factors needs to be restored. wilate® contains VWF and FVIII in a physiological 1:1 activity ratio to simultaneously restore the activity of both clotting factors, and facilitate simple and predictable dosing.2, 3
Many people with von Willebrand disease also have low levels of FVIII, which also needs to be corrected in order to restore normal haemostasis1
wilate® contains VWF and FVIII in a 1:1 ratio*, similar to the ratio found in the blood of healthy individuals3
*Based on VWF and FVIII activity levels. FVIII, factor VIII; VWD, von Willebrand disease; VWF, von Willebrand factor.
Because of the predictable decay of VWF and FVIII in wilate®, coagulation activity can be monitored using either VWF or FVIII levels
The ease of monitoring may help to ensure efficacy and tolerability of wilate® even after repeat dosing2

*Based on von Willebrand factor and factor VIII activity levels. FVIII: factor VIII; VWF: von Willebrand factor.
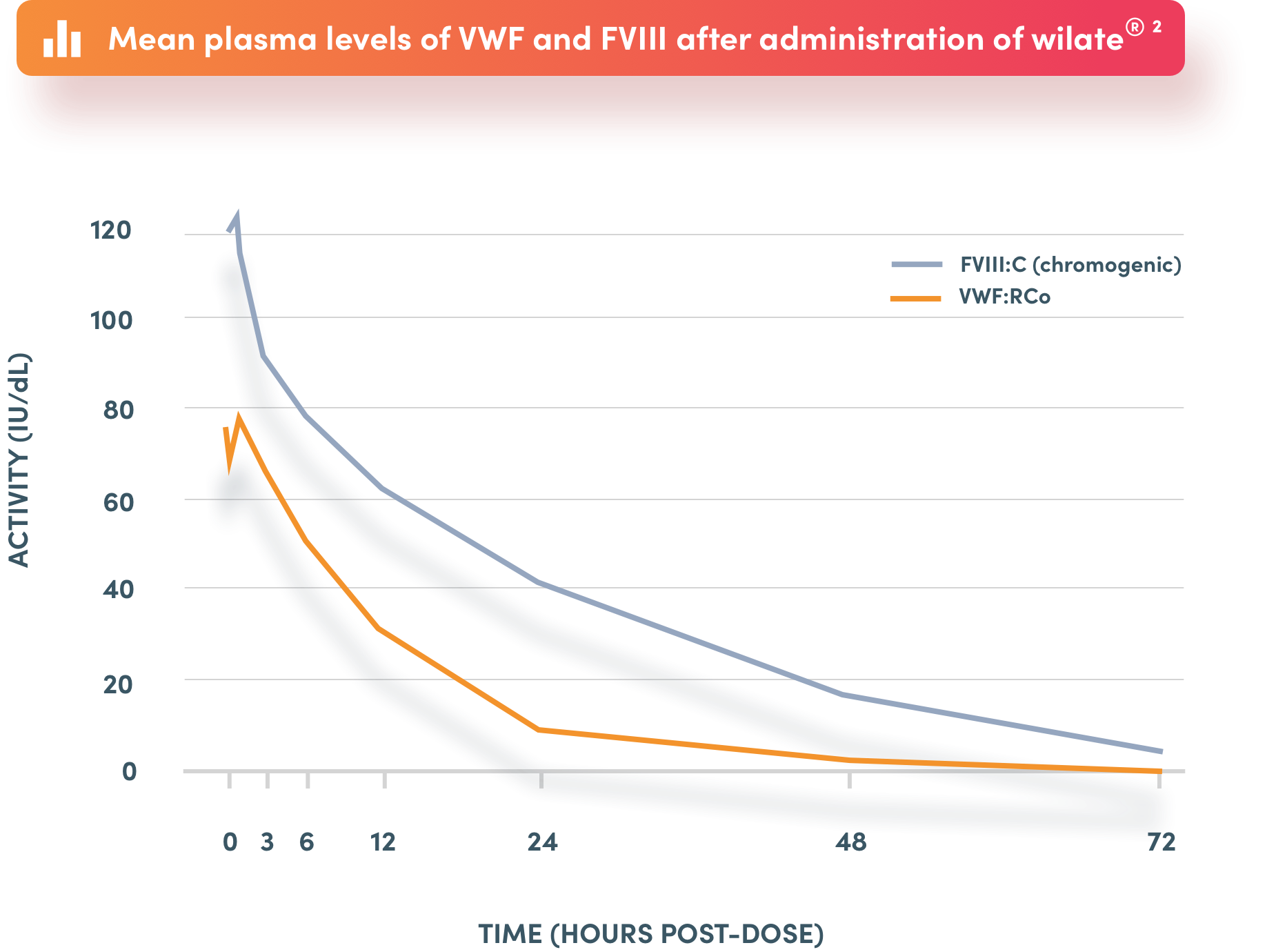
Parallel decay of VWF:RCo and FVIII:C
Simple dosing and monitoring using either VWF or FVIII levels
FVIII:C: FVIII clotting activity; FVIII: factor VIII; VWF:Ag: VWF antigen; VWF:RCo: von Willebrand Ristocetin Cofactor; VWF: von Willebrand factor.
Each wilate® package contains a vial of wilate® powder, a vial of sterile water*, a nextaro® transfer device and an infusion set.
The wilate® powder dissolves rapidly and the nextaro® transfer device allows quick and easy mixing to help save time during administration.
wilate® may be infused at a rate of 2–3 mL per minute.
*Includes 0.1% Polysorbate 80.
Recommended dosing for wilate®
*An initial dose of 50–80 IU/kg may be required, especially in patients with VWD type 3. †In some cases, such as in patients with gastrointestinal bleeds, higher doses may be necessary. FVIII:C: factor FVIII clotting activity for consistency; VWF:RCo: von Willebrand Ristocetin Cofactor.
Surgery
VWF:RCo target level: ≥60 IU/dL
FVIII:C target level: ≥40 IU/dL
An appropriate dose should be re-administered every 12-24 hours of treatment. The dose and duration of the treatment depend on the clinical status of the patient, the type and severity of bleeding, and both VWF:RCo and FVIII:C levels
On-demand treatment
20–50 IU/kg* to achieve adequate haemostasis
Prophylaxis
20–40 IU/kg
2 or 3 times per week†
wilate® is available in vials of 500 IU and 1000 IU. Each vial contains von Willebrand factor and factor VIII in a 1:1 ratio*.

*Based on von Willebrand factor and factor VIII activity levels.

The information on this website is not country specific, and may contain information that is outside the approved indications in the country in which you are located. Please contact your local Octapharma representative for the latest information specific to your country.
Please contact your local Octapharma representative for local prescribing information via our contact form.
IMPORTANT
The information on this website is based on the European Summary of Product Characteristics (EU SmPC). US HCPs and other country-specific websites: Below is a list of countries that host a local wilate® website based on local approved information and in local language. Click on the country link to be redirected to the local wilate® website.
The website is provided by Octapharma AG, Seidenstrasse 2, 8853 Lachen, Switzerland
www.octapharma.com
© 2022 Octapharma AG